On Pharmaceutical and Medical Malfeasance
Jun 19, 2021
An essay on science and its abuse by moneyed interests which encompasses both the industry and the government
Introduction
Is curing disease a sustainable business model? This very memorable question was posed by Goldman Sachs in a report done on April 10 of 2018 [1]. The details of the report aren’t as important as the general spirit that inspired the question, that sentiment being one of greed and irresponsibility. In this essay I will explore the manifestation of this spirit in the pharmaceutical and medical industry of the West. Specifically I will go over Primodos, Thalidomide, Tobacco, Sugar and Insulin, Oxycontin, and Atrizine. This broadly covers the food and drug industries’ with hands in birth control, recreational drugs, antidiabetics, painkillers, and agriculture. I will do my best not to assign malice where ineptitude may serve as a better explanation, but it is difficult not to be cynical with these matters.
Primodos and Thalidomide
Primodos will be the first of the pharmaceuticals that we will examined. It was manufactured by Schering AG and used from 1958 to 1978 in the UK as a hormone pregnancy test (HPT), being prescribed to around 1.5 million women. The drug consists of 20 μg of oestrogen ethinylestradiol and a large dose (about 10 mg) of progestogen norethisterone, which is 40 times the amount that is used today in oral contraceptives. At the time, it was more attractive than alternatives like Hagben which was more expensive and took 2 weeks for a result. Primodos also worked through an extremely simple mechanism: if you bled (typically within 3-6 days) you were not pregnant, and if you did not bleed you were pregnant. However, it had the horrible side effect of increasing the chance of congenital malformations, which in retrospect would have been the first thing they should’ve tested given that it was marketed as a HPT.
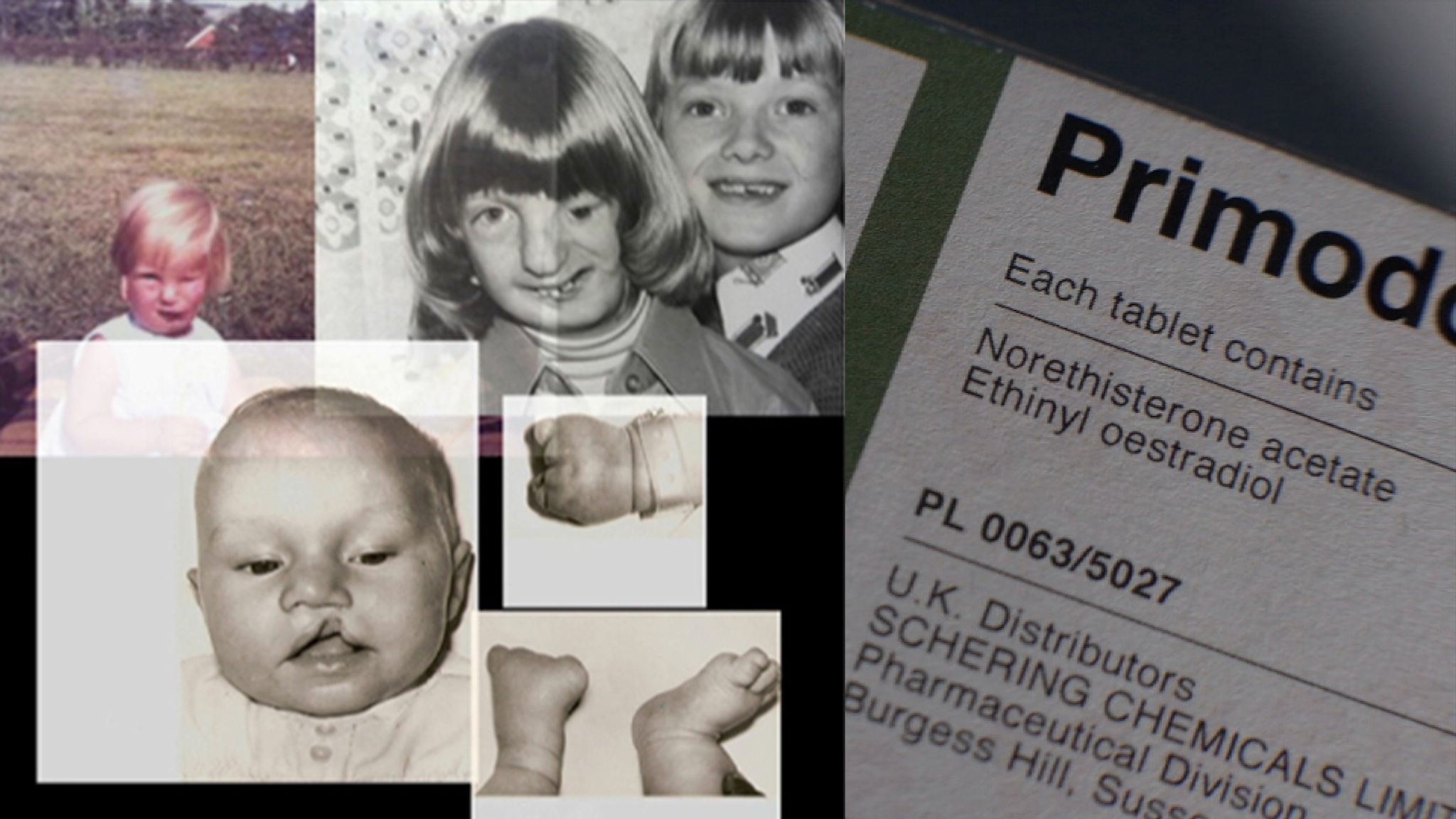
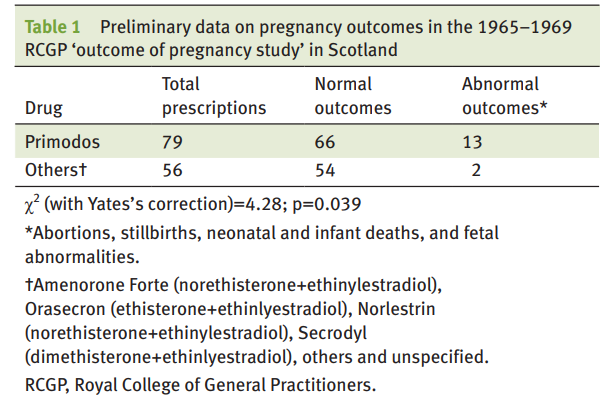
However, it wasn’t until after 10 years of use that the first evidence was published by Isabel Gal who presented evidence that HPTs increased the likelihood of spinal bifida, a birth defect of the spine and spinal cord. A year later in 1968, an additional study by statistician Dr Dennis Cook showed a correlational relationship between “the incidence of birth malformations” in the UK and the introduction of Primodos [2]. The Royal College of General Practitioners (RCGP) also conducted a study that started in 1965 and ended in 1969 recorded a much higher incidence of abnormal outcomes of pregnancies when using HPTs. In 1970 Primodos was no longer recommended for pregnancy testing but continued to use it as a treatment for irregular periods. Despite this change in indication, doctors were not explicitly warned until later in 1975 when “the possible risk of congenital anomalies was added to the label, as was a contraindication in pregnancy” [3]. In June of the same year a public notice was sent to all doctors in the UK advising that there may be a link between birth defects and the drug, recommending that the drug “should not now be normally used” [3]. In 1977 this changed to HPTs “should not be used” [3], and finally a year later Primodos was removed from the UK. From the first study in 1967, it took 8 years for a warning to be issued, and another 3 after that for it to be removed from the market. Why?
It is because the evidence was waved off both by Schering (now under Bayer AG) and the main regulatory body Committee on Safety and Drugs (CSD). In a memo dated to 6 June 1968, Schering UK notified to Schering Germany on their concerns about the promotion of Primodos as a pregnancy indicator, and a year later in 22 July 1969 they recommended to remove the pregnancy testing indication. This passed in 1970 yet many women continued to use it without realizing there was this change in recommendation. Additional documents were discovered in an investigation done in 2017 by Sky News who found that in 1975 Schering was notified by Professor William Inman, a Senior Medical Officer of the Adverse Reactions subcommittee of the CSD, had evidence to show that women taking HPT “had a relative five-to-one risk of giving birth to a child with malformations”.
Even prior to 1975, Inman and the CSD were consistently exposed to evidence about the risks associated with Primodos, being in correspondence with Isabel Gal and aware of her work even before it was published. Gal had even shared information on the RCGP study with him in 1969 to which he describes that “these did not produce any concrete results and it is somewhat difficult to summon up enough enthusiasm to place a high priority on this when so much other and possibly more important work is pressing.” He writes in his autobiography that he was aware that the HPT had hormones that were used had “important applications in the treatment of various gynaecological disturbances” and that they also happened to be the same hormones that were used for contraception [4]. The CSD were further exposed to their own studies confirming their suspicions in 1970, 1973, and 1975 yet did not do anything until mounting public pressure induced them to take action. By 1975, Gal had spent 8 years in correspondence with Inman and the CSD attempting to convince them to regulate HPT use. Although Dr. Inman is most well known for his accomplishments as a pioneer of risk assessment of drugs, it appears that in this case he did not feel pressed to do his part to prevent what could be thousands (at minimum) of birth defects if we take the rate of abnormal outcomes to be similar to the rate found by the RCGP study (16.4%). There was at least 700 claims as of October of 1978 [5].
More recently in 2017, an expert working group (EWG) of the UK Commission on Human Medicines, the main regulatory agency of the UK, concluded that “there was no clear evidence that taking Primodos during the first trimester of pregnancy could cause congenital anomalies via a direct pharmacological action” but that such an effect could not be excluded. Later in 2018, the EWG also studied the Zebrafish study by Brown et al [6] as well as the meta-analyses by Heneghan et al [7], the former asserting a definitive causal link between congenital defects and primodos in zebrafish, and the latter concluding an increased risk in humans. In light of all the previous evidence, the study by Heneghan et al. which found that in 26 studies which included 71,330 women (4209 of whom were pregnant) that found a 1.4x odds ratio of increased risk of all congenital malformations [7], it is hard to believe that they could not find evidence. Rather they were unwilling to accept the evidence, which is in line with what this working group had said critiquing a meta-analyses done by Heneghan. “Therefore, the quality of most studies used is questioned and, as a result, the conclusions of the meta-analysis cannot be considered reliable.” [5]
Thalidomide (aka Contergan) was a very similar case to Primodos, but its fall from grace was a much more publicized event. Initially marketed by Grünenthal as a “wonder drug” for insomnia, coughs, morning sickness, colds, and headaches, Thalidomide later became implicated in the death of approximately 2000 children, and the genetic malformations of more than 10,000 [8]. It was most prevalently used in Germany where it was on the market for five years between 1957 and 1961 before the reports of Widukind Lenz and William McBride prompted Grünenthal to take the drug off the market. “Cases of deformities were multiplying in Lenz’s office in the city of Hamburg as well as in McBride’s hospital in Australia. Both doctors attempted to find the causes and, independently, came to the assumption that Thalidomide could be a possible cause.” By this point thalidomide had been marketed to 46 countries being sold 300 million times [9].

Fortunately, it was never sold publicly in the USA as Frances Oldham Kelsey of the FDA refused approval to Richardson-Merrel 6 times [10]. The possibility of it was certainly there as there were two avenues through which it could have entered, via Richardson-Merrell (now a part of Sanofi) or Smith, Kline & French (now GlaxoSmithKline or GSK). The latter declined to commercialize the drug after conducting their own studies in 1956-1957 and concluding that it was not effective. The former, however, applied for FDA approval in September 1960, but was continuously denied by Kelsey due to its lack of studies and reliance on testimonials. The representatives from the industry end were getting increasingly frustrated because as Kelsey reports, “They were particularly disappointed because Christmas is apparently the season for sedatives and hypnotics (sleeping pills). They kept calling me, and then just came right out and said, ‘We want to get this drug on the market before Christmas, because that is when our best sales are.” [10] It was only in March 1962, almost half a year after Thalidomide was pulled from the German market due to the Scandal, did Richardson-Merrel withdraw its application.
Just a month prior in February of 1962, a letter (shown in Figure 1 below) was sent from RIchardson-Merrell to doctors saying that there is “still no positive proof of a casual relationship between the use of thalidomide during pregnancy and malformations in the newborn” [11]. Later in September of the same year the FDA concluded that Richardson-Merrell had acted illegally in the promotion of the drug before it was even approved. Activities included the industry standard; hiring influential doctors to promote thalidomide, and helping researchers and scientists write articles in favor of thalidomide. It is also worthwhile to note that the drug was widely distributed by Richardson-Merrell before it was ever approved. More than 2.5 million tablets were given to “1,000 doctors throughout the United States on what was called an investigational basis. The doctors, in turn, gave thalidomide to nearly 20,000 patients, several hundred of whom were pregnant women.” [10] This resulted in at least 17 American children born with thalidomide induced deformities but that number is likely higher since not everyone who took the drug may have been aware its connection to a deformity.

Tobacco Industry
This is probably the least controversial of the ones that I will cover in this essay as it is virtually undisputed that the tobacco industry did their best in the latter half of the 20th century to obfuscate the link between lung cancer and tobacco products. It is by far the most publicized and widely known of these examples and serves as the model of the tight-knit relationship between science and the corporations that manipulate it for their own gain. As late as 1994, the executives of the tobacco industries used periphrastic language to claim that
- Cigarettes were not addictive
- Nicotine is not addictive
- Smoking was not proven to cause cancer
such as in the case of James Johnston (Of R.J. Reynolds) saying “that cigarettes and nicotine do not meet classic definition of addiction” [13].
In reality, the industry had long been aware of the addictability of their products and the risks associated with cancer. As early as the 1950s, both tobacco scientists and lawyers recognized the health problems associated with smoking and were hopeful that with enough research and funding they would be able to develop a product that reduces the health risks. A snippet from an internal memo written in the 1950s from a lawyer at PR firm Hill & Knowlton evokes this naïve sentiment regarding cigarettes “Boy! Wouldn’t it be wonderful if our company was first to produce a cancer-free cigarette. What we could do to the competition” [14]. Despite the internal recognition of the risks, the public face of tobacco was always on the defensive, denying or dismissing evidence not in their favor.
In a more egregious example of a “conspiracy” US tobacco executives met at New York’s Plaza Hotelin December 15, 1953 to discuss how they were manage public perception in reaction to the growing public consciousness of the link between cancer and tobacco [15]. In their meeting notes they admit that, although they were not allowed to form a trade association due to anti-trust laws, they wanted to informally meet to create a strategy to promote their interests. Later, the Roper Proposal written in 1972 by Fred Panzer, the VP of the Tobacco Institute (lobbying association for the US tobacco industry), presented the strategy of the corporations against litigation and public opinion, which was to sow doubt about the health risks of tobacco without denying it, advocate for the publics autonomy to choose whether to smoke, and encourage further scientific research to resolve the question. The memo also showed that this strategy was chosen to delay, rather than turn the tide of public opinion as they perceived that it was only prolonging the inevitable. However, Panzer proposed a way out of this quagmire, presenting two options; the Constitution hypothesis, and the Multi-factorial hypothesis [16].
The former was the hypothesis that “people who smoke tend to differ importantly from people who do not, in their heredity, in constitution makeup, in patterns of life , and in the pressure under which they live.” It was first proposed by Carl Seltzer while working with Frederick Stare in the Harvard School of Public Health. The Multi-factorial hypothesis posited that “as science advances, more and more factors come under suspicion as contributing to the illnesses for which smoking is blamed – air pollution, viruses, food additives, occupational hazards and stresses.” Finally, Panzer advocated for the use of “experts to consult on the design of the study”, present it to key groups (the Senate, House, Cabinet, White House, State Governors, Medical School & University Professors, scientific bodies), write a book and publish it in a “legitimate house” and market it in as many ways as possible. In short, they wanted to blame everything except cigarettes for the illnesses that smokers ended up contracting, and they would legitimize this through the use of scientific experts (such as Gary Huber, Carl Seltzer, and Frederick Stare of Harvard) and specialists who would support them and the widespread broadcasting of their experts to the public as legitimate.
Sugar, Fat, Heart Disease and Diabetes
This section will be dedicated to what I believe will be the biggest health scandal in the coming decades, which is that the food industry, the academy, and the government (mainly the USDA and FDA) wrongly attacked fat as the main driver of heart disease. In doing so, it promoted the marketing of low-fat/no-fat foods, which resulted in the near ubiquitous addition of sugar into food products. Excess sugar is arguably worse than excess fat in one’s diet hence this may have inadvertently worsened the current obesity crisis and all of its associated health problems. Typically, when a high-fat food such as yogurt has its fat content removed, food manufacturers have to compensate for the lack of richness and so lots of sugar is added. For example, 6 oz of low fat yogurt shown below has 13 grams of added sugar, which is about a third of what is in a coke can. The demonization of saturated fat has also resulted in the near abandonment of butter and lard for polyunsaturated fats like vegetable oil, and soybean oil, both of which, according to some studies, may be detrimental to human health.

The origin of this finger pointing can be traced to a study done by Ancel Keys called the Seven Countries Study (SCS) which showed a correlation between saturated fat and heart disease [17]. In combination with the David Mark Hegsted’s Hegstead Equation,which predicted that dietary saturated fat and cholesterol (primarily from meats and eggs) raised cholesterol levels, it created the perfect medium for the sugar industry to create a narrative that the consumption of sugar was perfectly healthy despite early warning signs in the 1950s that showed links between coronary heart disease and sucrose (ie sugar). To be clear, there are many misconceptions about the SCS which I will not get into in this essay, but it should be noted that the study was not likely to have been conducted in bad faith, but other forces at hand may have used the SCS conclusions to push for an anti-fat campaign. The SCS doesn’t even implicate fat in general but rather a specific kind of fat: saturated fat. Higher amounts of non-saturated fat in diets tracked by the SCS was not even associated with heart disease. However, the message appeared to be lost, and a crusade was waged against all forms dietary fat and cholesterol to fight heart disease.
After thorough analysis of internal documents of the Sugar Research Foundation (SRF), the largest sugar lobbying group, Cristin E. Kearns of the University of California concluded that evidence suggests that “the industry sponsored a research program in the 1960s and 1970s that successfully cast doubt about the hazards of sucrose while promoting fat as the dietary culprit in CHD” [18]. As early as the 1950s, the SRF devised of a strategy to “increase sugar’s market share by getting Americans to eat a lower-fat diet”. An example of this was Project 226 which discreetly funded Harvard scientists, providing financial support towards esteemed member of the scientific community like Harvard’s Dr. Mark Hegsted, Dr. Robert McGandy, and Dr. Fredrick J. Stare (chair of Harvard’s school of Public Health who was mentioned earlier). They went on to produce a literature review in 1967 Dietary Fats, Carbohydrates and Atherosclerotic Disease that was published in the New England Journal of Medicine (NEJM). The NEJM paper deemed it a matter of “responsibility and opportunity for the food industry” to lessen “the development of atherosclerosis” by changing the diet to include less saturated fat and dietary cholesterol while considering sugar to be of minimal risk [19].
Frederick J. Stare was arguably the most important nutritionist of the 20th century, which may explain why his NEJM was influential. He found the nutrition department at Harvard; coauthored a nutrition manual for medical students; coauthored a textbook for udnergraduate nutrition students, became editor for multiple decades of the Harvard nutrition editorial Nutrition Reviews. His work and expertise were relied upon by the Food Protection Comittee (FPC) of the National Research Council and the National Academy of Sciences when the government was evaluating DDT in 1950. However, he had a track record of defending industry products, fervently maintaining that additives, sugar, and processed foods were the future. He maintained on the NYT that “There has never been one medically documented death due to proper use of insecticides.” Obviously he was wrong about DDT, but he was also wrong about sugary drinks, being an advocate for coca-cola as a good source of phosphate. His associate Dr. Elizabeth M. Whelan and coauthor of Panic in the Pantry was a frequent promoter of processed foods like cereal and Kool-aid to children. She was quoted to have said “The availability of something like soft drinks to children does not pose any known health hazard that would harm them”. Hm… does this have anything to do with why 1 in 5 children in America are obese? [20]
The industry also suppressed research that would have cast aspersions towards sugar and undermine sugar’s public perception and hence their financial interests. For example, a 1969 study funded by the SRF called Project 259 called the Dietary Carbohydrate and Blood Lipids in Germ-Free Rats linked the consumption of sucrose to elevated triglyceride levels (typically associated with heart disease) and bladder cancer. Due to its unfavorably conclusions towards sugar and its implications for human health, the SRF terminated funding for the study, and Dr. W.F.R. Pover, the main researcher, was unable to finish and publish his study due to the lack of funding [21].
Despite the long-term changes in macronutrient composition with a heavier emphasis on carbohydrate at the expense of protein and fat consumption, obesity and heart disease continues to grow…. This can be clearly seen in three studies, one in 1971-2000 by the CDC which tracked an increase in obesity from 14.5% to 30.9% with less fat and protein consumption and increasing carbohydrate consumption (from 42.4% to 49.0% of the diet for men and from 45.4% to 51.6% for women)[22]. Similar observations were found in a study done by Austin, Ogden and Hill that found increase in obesity from “11.9% to 33.4% in men and from 16.6% to 36.5% in women” in the time period of 1971-2006 despite changes in diet composition. During that time, the “percentage of energy from carbohydrates increased from 44.0% to 48.7%, the percentage of energy from fat decreased from 36.6% to 33.7%, and the percentage of energy from protein decreased from 16.5% to 15.7%” [23]. Finally, we get to the most damning of the studies which calls this phenomena “The American Paradox” [24]. Using a population survey that constituted nationally representative samples, Dr. Adrian Heidi concludes that “reduced fat and calorie intake use of low-calorie food products have been associated with a paradoxical increase in the prevalence of obesity.”
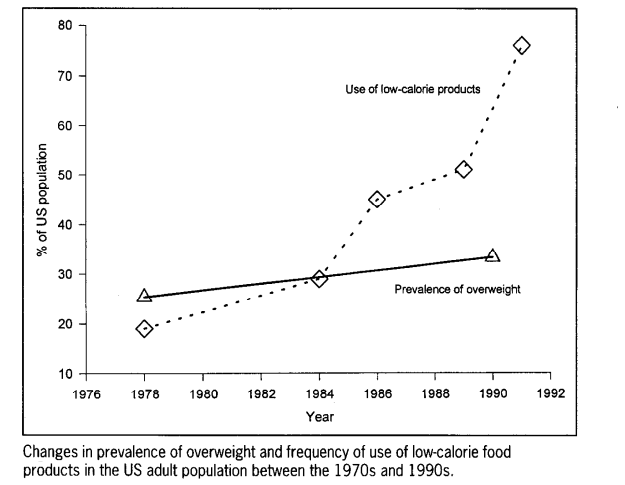
Add this to the fact that saturated fat consumption has also gone down (from 13%~ to 11%~), while heart disease has more than doubled from 1979 to 2004 (increase from 1,274,000 cases/222 million to 3,860,000 cases/292 million which is about 2.3x higher rate) [25]…. and we have a glaring problem on our hands, namely that the dietary paradigm that has been propagated seems to be at the very least inaccurate and incomplete, if not completely wrong altogether.
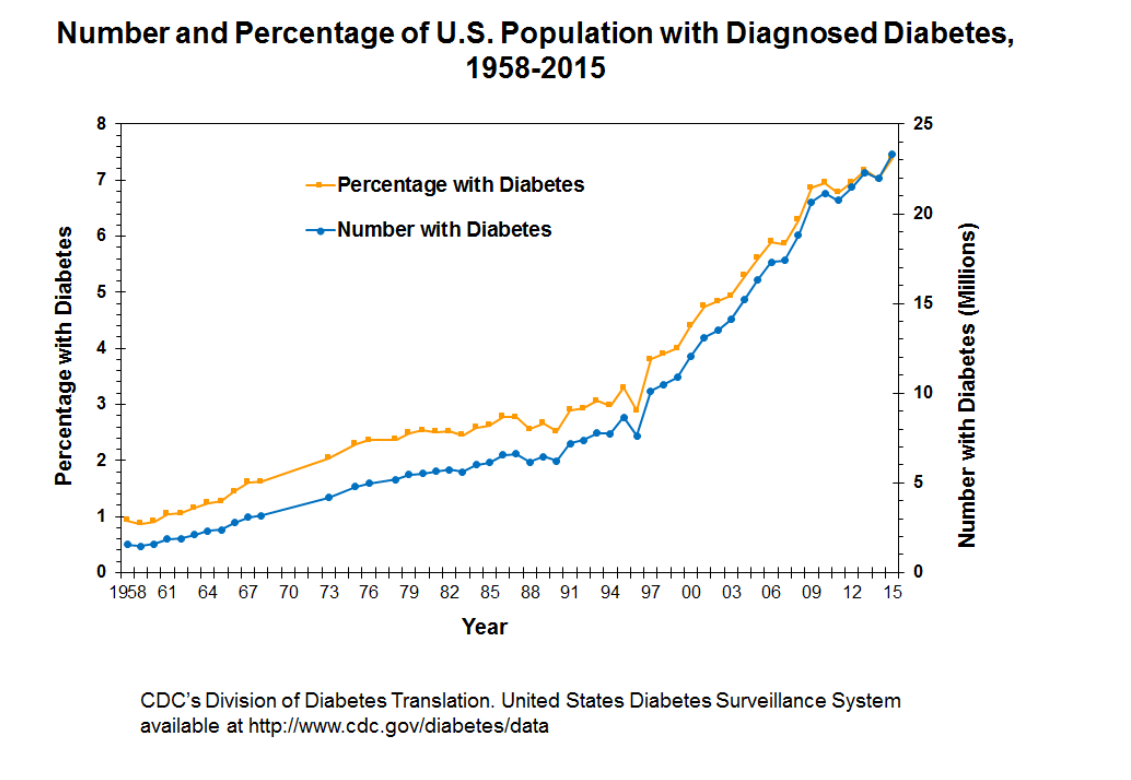
It is also important to account for the growing amount of diabetics (from 1% to 10% since the 1950s according to the CDC) in the US population who are entirely dependent on insulin as a matter of life or death due to insulin being the medical establishment’s only approved treatment for type 2 diabetes [26]. I believe there is a system in which the research from which policy and guidelines are developed are either purposely misleading, or unintentionally misleading the public with the result of possibly exacerbating the chronic illness in the US and other countries following in the example of the US. I do not ascribe malice to the part of the institutions being discussed here, yet it is entirely within the realm of possibility that there can be a financial incentive for the proliferation of disease and illness. Chronic illness that requires constant medication may be a much better “business” model than health that does not.
Today, the sugar industry (and food manufacturers as a larger category) appear to still employ similar tactics. An illustration of this is Coca-Cola providing close to 4 million dollars (2015) since 2008 to “Dr. Blair, a professor at the University of South Carolina whose research over the past 25 years has formed much of the basis of federal guidelines on physical activity, and Gregory A. Hand”, a former dean of the West Virginia University School of Public Health before Coca-Cola controversy forced him to resign (now Dean for the College of Health Professions at Wichita State Unversity), and Dr. Hill, Director of University of Alabama Nutrition Obesity Research Center [27]. These three are part of Global Energy Balance Network which promotes the argument that Americans are not paying enough attention to exercise and too much attention to diet [28]. I will do a larger article on food, chronic illness, and pharmaceutical companies later that will speak at length on things like sugar, soybean oil, and insulin that I’ve glanced over here…
Purdue and Oxycontin
Like tobacco, it is practically mainstream knowledge that deceptive tactics that were utilized by Purdue Pharmaceuticals and the Sacklers in the marketing of OxyContin® (controlled-release oxycodone hydrochloride) that began in 1995. It was so successfully marketed that within 5 years “of the drug’s launch, OxyContin® became the number-one prescribed Schedule II narcotic in the United States” and in the 2010s there were more than 250 million prescriptions of opioids [29]. At the peak there were 81.3 opioid prescriptions for every 100 people in America (many people had more than 1 prescription) [30]. This success was primarily due to it being marketed as being uniquely safe and effective, masking its real risk of abuse, addiction, overdose and death. Its initial claim to fame was that it was longest lasting narcotic pain reliever and due to its slow release mechanism it would be “safer” and less addictive, making it a superior choice for pain relief medication.
However the slow release mechanism was easily bypassed. If the oral tablet was crushed and snorted, instead of a slow release it would result in the rapid release of the drug with the possibility of overdose. And if not overdose it would very quickly lead to abuse and addiction. Hence a strategy abusers often adopted was “doctor shopping” in which a person seeking Oxycontin would go to various doctors complaining about pain so that several prescriptions can be obtained. This is supported by a study that states that “70% of OxyContin® abusers obtained the drug via a physician’s prescription” [31].When accounting for the fact that most people (80%) who use heroin first misused prescription opioids it is very easy to establish the connection between the over-prescription of OxyContin and the current opioid epidemic, which kills roughly 50,000 every year. Of the 50,000 overdose deaths, heroine claims roughly the same number of lives as prescription opioids do as seen in the figures below. [32]
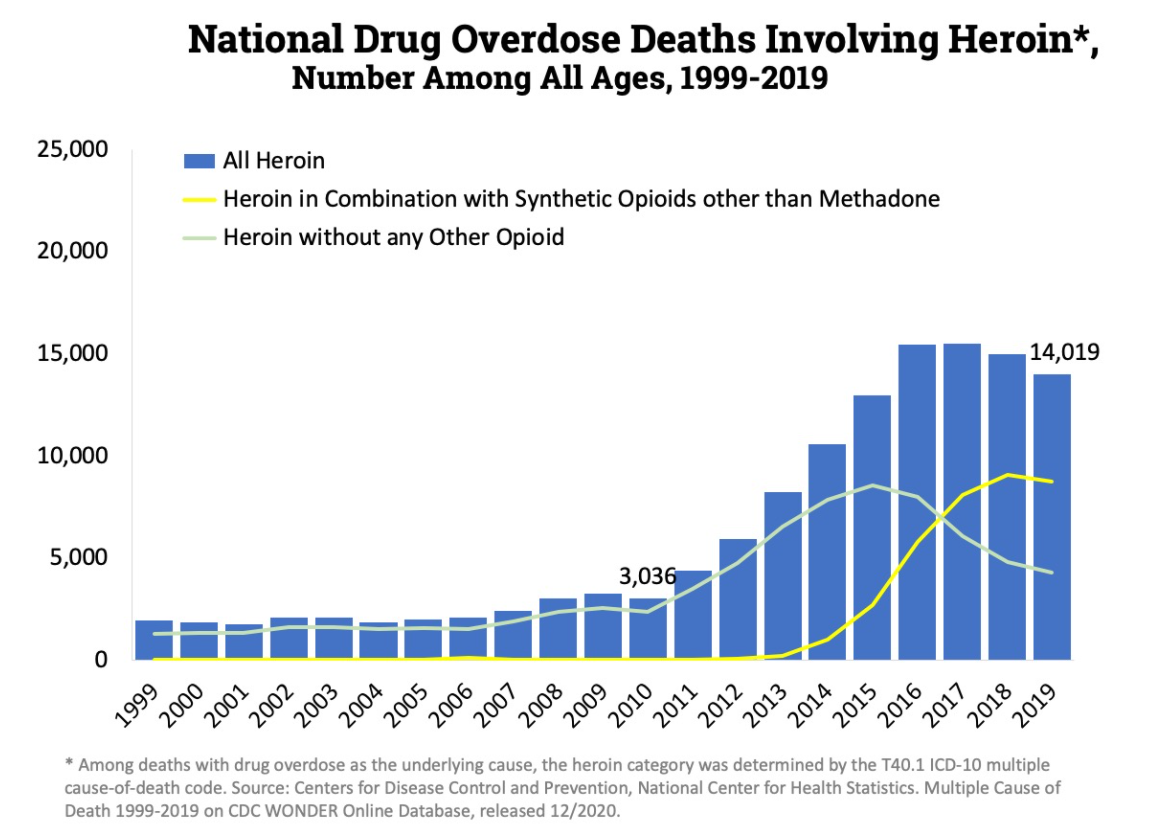
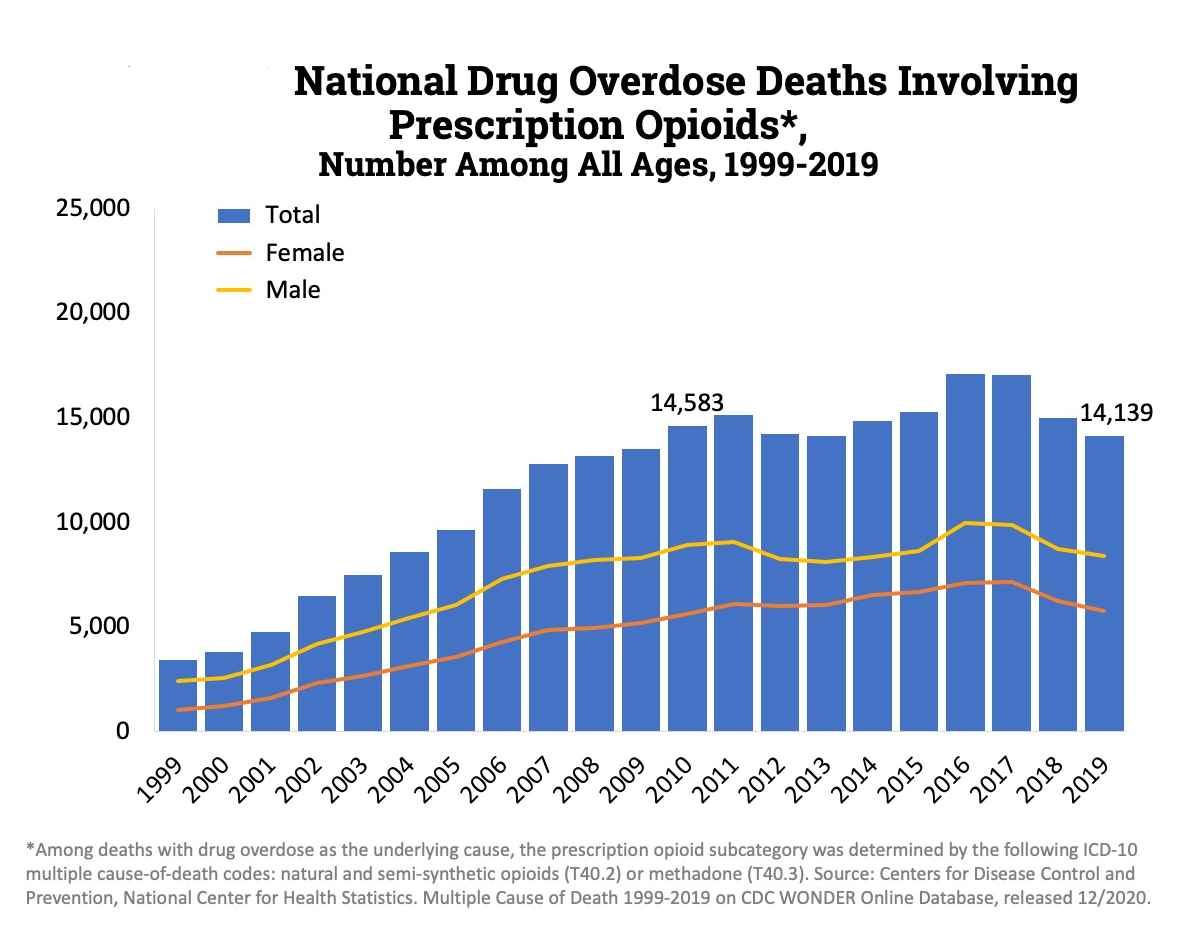
So what exactly did Purdue claim about Oxycontin? They famously marketed to doctors that
“less than 1% of patients taking opioids actually become addicted” and “that addiction to opioid medication is “rare””.
This was based on 3 studies that showed very little relevance upon even a cursory examination. The first by Medina and Diamond only had 2 of the 2639 patients studied taking Oxycontin because the purpose of the study was drug dependency for patients with chronic headaches or in other words, this study was not specifically designed to study Oxycontin. That study also showed a 19% dependency rate for those on narcotics (ie opioids) which is very different than 1%. The second study by Perry and Heidrich was a questionnaire sent to 151 burn facilities to review pain management for burn patients but it was conducted in 1982 and did not study anything regarding addiction. So it was irrelevant in two categories as the study did not apply to OxyContin, a slow-release narcotic released in 1995, nor did it have any application in determining how addictive opioids are. The last study referenced was a 110-word paragraph by Porter and Jick in 1980 which is so short I can just quote below.
“To the Editor: Recently, we examined our current files to determine the incidence of narcotic addiction in 39,946 hospitalized medical patients who were monitored consecutively. Although there were 11,882 patients who received at least one narcotic preparation, there were only four cases of reasonably well documented addiction in patients who had no history of addiction. The addiction was considered major in only one instance. The drugs implicated were meperidine in two patients, Percodan in one, and hydromorphone in one. We conclude that despite widespread use of narcotic drugs in hospitals, the development of addiction is rare in medical patients with no history of addiction.”
That study was flawed for several reasons. Firstly, it did not have any application for slow release oxycodone products like OxyContin as it was released in 1970. Secondly, the dose prescribed in the study was much lower than the doses used when prescribing OxyContin. The largest dose of Percodan (implicated in the study above) is equivalent to the lowest dose of OxyContin. Lastly, and most importantly is that the study only monitored short-term users of narcotics for pain management rather than people who are prescribed opioids for pain management, and hence are much more susceptible to tolerance and dependence from longer-term repeated use.
The actual rate of addiction , according to a meta-analyses done by Fishbain et al in 1992, is between 3.2% to 18.9%, while other papers report addiction rates as high as 34% in chronic pain patients. Purdue knew about this as yet did everything they could to continue to falsely advertise OxyContin. Hence from 1997 to 2001, there was no addiction warnings in the “Warning,” “Precautions,” and “Information for Patients/Caregivers” sections, nor were there contraindications for those with prior drug addictions. Similar Schedule II products in the market like MS Contin and Oramorph SR acknowledged addiction risk, and OxyContin, when marketed in places like Austria, also listed addiction warnings.
While the FDA did its best to regulate OxyContin and address the concerns of the public, it was often ineffective. There always seemed to be a way around it. For example, after negotiating with the FDA, Purdue modified their OxyContin label in 2002 to include a “black box warning”. This was the strongest warning regarding abuse risks found on prescription drugs. However, the warning, instead of acting as a deterrence, was used by Purdue to expand its usage in the management of pain to include post-operative pain. Purdue had seen this regulatory action by the FDA as an opportunity to expand its business . Purdue’s budget plan in 2002 explicitly lays this strategy out.
“The action taken by the FDA to clarify the OxyContin® Tablet labelling has created enormous opportunities. In effect, the FDA has expanded the indication for OxyContin® Tablets to any patient with moderate to severe around the clock persistent pain… This broad labelling is likely to never again be available for an opioid seeking FDA approval. This may give OxyContin® Tablets a competitive advantage”
Of special concern is the revolving door policy that is present in virtually all the industries (such as in media and as I had spoken of in another article). The very same FDA medical reviewer OxyContin in 1993, ended up joining Purdue as the Executive Director in Risk Assessment Coordination for New Products in 2003. This man was Dr. Curtis Wright who even by 2003 testified that he still believed addiction to Oxycontin was rare. Consider also the “independent” group of doctors and patients called the Partners Against Pain which appeared to be a grassroots movement that was promoting the use of OxyContin but was in actuality funded and controlled by Purdue. This group had the veneer of promoting public good and public health with solid science and tricked many doctors (and their patients) into buying the Purdue narrative. Hence, just like the other sections of this essay there is deception deployed to every level of the hierarchy; to the patients, the doctors, to scientists, and to the regulators.
Sygenta, Atrazine, and “Gay Frogs”

This last section will be likely the most fantastical of the topics we have already covered given that it is tied with Alex Jones’ famous quote and accusation that “they are putting chemicals in the water that are turning the friggin frogs gay”. This is mainly based off of a documentary made by a YouTuber who runs a channel called “Oki’s Weird Stories”. He did an extremely thorough investigation, personally calling and interviewing the individuals involved in this controversy including 3 EPA employees involved in the first EPA review of atrazine in 2003 [33]. The bulk of this section will be based off of his video and the sources that he uses.
First… What is Atrazine? It is an herbicide produced by Sygenta used on broadleaf weeds in crops like corn, sugarcane, sorghum, turf and residential lawns. It enters the environment through runoff and is frequently detected in “US streams, reservoirs, groundwater, and precipitation” to the point where it was considered the most commonly detected pesticide detected in drinking water sources, being detected in 30% of 2460 ground water sites surveyed in a study done in 2000 (Pesticides of US 1992-1996) [34][35][36]. It is considered non-toxic by the EPA for mammals, but toxic to fish and aquatic plants in concentrations as low as 20 μg/l, and amphibians in concentrations from 200 to 127,000 μg/l. Two studies done by Tyrone Hayes from UC Berkeley in 2002 found that atrazine exposure induces hermaphroditism (showing both testes and ovaries) in male frogs, causing impaired sexual development and skewed sex ratios [37]. They also saw a ten-fold decrease in testosterone levels in frogs exposed to atrazine levels 1/30th the safe level set by the EPA for drinking water. These observations were theorized to be factors contributing to the global amphibian population declines. They may also have implications into human health given their prevalence in our drinking water.
Hayes continues to publish his work in scientific journals on this phenomena, and it seems that his observations about atrazine’s endocrine disrupting effects are being cited and replicated by other members of the scientific community. However, his work could not be backed by the single most important body when Atrazine was evaluated in 2003 and 2007; the EPA. Oki noticed this discrepancy and contacted and interviewed three scientists who were on the EPA risk assessment panel during the evaluation of Atrazine; Daniel Schlenk, Jason Rohr, David K. Skelly. In his interview with them, Shlenk says that
“Do I think that Syngenta was concerned about the PR outfall? I-I cannot tell you but I can tell you what they did and what they did was they tried to really destroy his career in my opinion. And I think that was really wrong. It totally affected the way he did science. Totally affected his lab, I mean I don’t think he took any graduate students after that. Cause he was afraid for what that would, you know, would do their careers because they came out of his lab”.
Even before the first breakout paper by Hayes in 2002, there were studies showing the possible effects of atrazine on amphibian gonadal development [38][39]. This may explain why Ecorisk had actually reached out to Hayes in 1997 to contract him to do research for Syngenta on atrazine effects on amphibians. Soon however the two parties began to run into problems. This was because the results that Hayes had found were not favorable toward Sygenta as Hayes found the exact same thing that he would find later in 2002, namely hermaphroditism due to atrazine exposure. Thus, Syngenta began employing the standard industry practices. As previously mentioned, these include cutting funding, stalling progress, bringing in their own experts to nitpick his data, refusing to share his findings with the EPA. Hayes even states that they asked him to manipulate the data to “make it go away” to which he responded with leaving the contract in 2000 [33].
After the 2002 study, Syngenta went into the next step of their strategy which was to counter Hayes’ findings with their own findings through funding their own research. Not surprisingly, they claimed to have found no such relationship between atrazine exposure and hermaphroditism despite a study done by Dr. James Carr replicating Hayes’ results albeit at higher atrazine concentrations. Despite this Carr was quoted in a Syngenta press conference to have said “We have been unable to reproduce the low-concentration effects of atrazine on amphibians reported elsewhere in the scientific literature” [40]. In 2004, there were 5 independent labs that had come to a conclusion that atrazine affected gonadal development (Hayes, Miyahara et al. 2003, Reeder et al. 1998, Parshley 2000, Tavera-Mendonza et al. 2002), while ALL of the studies publishing contrary conclusions were conducted by Ecorisk on behalf of Sygenta and involving the same scientists (Ecorisk panel members Carr, Giesy, Ronald Kendall, Ernest Smith, Keith Solomon, and Glen Van Der Kraak) [40].
In the face of all this what did the EPA do? It conducted a risk assessment for Atrazine in 2003 which was finalized in 2007. They needed to answer this question: At what concentrations does atrazine have adverse effects on amphibians and is it below ecologically relevant levels? In the 2003 review, the EPA performed a literature review comprising of 17 studies, of which 12 were funded by Syngenta with only two finding no adverse effects. So of the 17, 15 were found to have supported the initial charge against Atrazine. Thus in 2003, EPA concluded that there was sufficient enough evidence to form a hypothesis but more studies were required, specifically those that could replicate Hayes findings. Who was asked to do this? Sygenta of course. Sygenta was also tasked with developing “draft testing protocols to the Agency for review”, meaning Sygenta would not only do their own studies, but also develop protocols that other scientists must also follow when doing studies on atrazine and amphibians.
Under such protocols, all of the literature that implicated atrazine as an endocrine disruptor were retroactively thrown out because they did not meet the good laboratory practices as determined by Sygenta. In the final conclusion in 2007, only 1 study (Kloas et al. 2009) met the criteria established by the EPA (and Sygenta), and it happened to be a study funded by Sygenta that concluded that atrazine was not associated with any harm to amphibians [41]. In the final report of 2007 the EPA published that
“Based on the available data provided by the DCI studies, the Agency has concluded that atrazine does not adversely affect amphibian gonadal development. The Agency has further concluded that no additional studies are required to address the hypothesis that atrazine adversely affects amphibian gonadal development.” [42]
Fortunately, in more recent times, the EPA has retracted their statement and now are more “agnostic” stating that in their updated literature review which includes 55 studies (as opposed to just one), they have found “there is potential for chronic risks to amphibians” [43].
We’ll end with a list of things that Sygenta considered doing to Tyrone Hayes in their campaign against him and his work. Claire Howard found court documents through the Freedom of Information Act in 2013 giving us a glimpse into their stratagem [44].
- Investigate his wife and tap his phone
- Psychologically profile him and paint him as “paranoid schizo and narcissistic”
- Implicate him in a scandal so that “enviros will drop him”
- Contact UC Berkeley & Academics
- Create negative publicity about him from 3rd parties
- Cut him in on unlimited research funds
- Purchasing paid results shown on search engines when searching for Tyrone Hayes’ name. (They actually did this one..)
What they ended up doing was moving massive amounts of resources, both financial and social to wage a PR campaign (hope this sounds familiar by now) to rehabilitate atrazine or obfuscate the truth. Like the Tobacco industry and their use of “experts” they payed a group of 130 people and groups to appear as neutral third-party supporters of Atrazine. For example, Don Coursey, Ameritech Professor of Public Policy at the University of Chicago could be relied on for a hefty price of $500 an hour. Clare Howard reports that “at least four public relations firms were hired to work on the Syngenta campaign”, primarily the White House Writers Group and Jayne Thompson & Associates [45]. Interestingly, in their memo to the White House Writers Group, one of the strategies was to revise the wikipedia page for Atrazine. The group was also were payed to write op-eds in favor of atrazine, and were specifically told to avoid language suggestive of their connection to Sygenta. This along with their purchasing of search engine results should be worrying as we are often dependent on our search engines and resources like Wikipedia for our information. If it is possible to control what we see (or don’t see) then it is possible to dictate what is true and what is false.
Conclusion
So what are commons strategies that were used by the companies and industries I have referenced above?
- Fund and pay for research that supports your products
- Gaslight by denying or discrediting detractors
- Astroturf by setting up “grassroots” organizations and funding “independent” writers for op-eds
- Delay regulation as long as possible or work with regulation if it can work in your favor
- Revise information available to the public by buying search engine results or changing wikipedia pages
By now I hope that you can see the threads of the pattern here which is that under the current framework in 2021, which fundamentally hasn’t changed from the mistakes of the past, there is no guarantee that what you understand to be safe and effective today may be determined to be hazardous to your health tomorrow (or vice-versa). By nature of what science is, which is a continual process through which we discover knowledge, the current paradigms, no matter how well-established they may appear, can always be challenged (both by industry agents like the Tobacco industry or by those outsiders like Gal). Ultimately, science just like media, politics, culture can be molded to re-tell a narrative with enough money and influence thrown at it. After all, as long as there are humans around, there will be people subject to corruption. And once a narrative germinates in public consciousness it is very difficult to unroot especially if there are people with fancy degrees from esteemed universities and lofty positions of prestige propagating the narrative.
References
[1] Goldman Sachs asks in biotech research report: ‘Is curing patients a sustainable business model?’
Primodos & Thalidomide
[2]Primodos the Secret Drug Scandal
[3]Forgotten Primodos story and the roles of general practitioners
[4]First Do No Harm The report of the Independent Medicines and Medical Devices Safety Review
[8]THE THALIDOMIDE TRAGEDY: LESSONS FOR DRUG SAFETY AND REGULATION
[9]The Thalidomide tragedy in Germany
[10]Frances Oldham Kelsey: FDA Medical Reviewer Leaves Her Mark on History
[11]The Story of Thalidomide in the U.S., Told Through Documents
[12]US lawsuit extends thalidomide’s reach
Tobacco
[13]Inside the Tobacco Deal - Congressional Hearings of 1994
[14]REENGINEERING THE CIGARETTE
[15]Background Material on Cigarette Industry Client - December 15, 1953
[16]Roper Proposal
Additional Reading
Fat and Sugar
[17]Ancel Keys and the Seven Countries Study:An Evidence-based Resposne to REvisionist Histories
[19]Dietary Fats, Carbohydrates and Atherosclerotic Disease
[20]Harvard’s Sugar-Pushing Nutritionist
[23]Divergent trends in obesity and fat intake patterns: the American paradox
[24]Trends in Intake of Energy and Macronutrients
[25]Heart failure-related hospitalization in the U.S., 1979 to 2004
[26]Long-term Trends in Diabetes April 2017
[28]Coca-Cola funds scientists who shift blame away from bad diets
Additional Reading
[]How the Sugar Industry shifted blame to fat
[]Sugar Industry long downplayed potential harms of sugar
[]The FDA Orders a Total Cyclamate Ban
OxyContin
[29]The marketing of OxyContin®: A cautionary tale
[30]U.S. Opioid Dispensing Rate Maps
[31]OxyContin® Abuse and Overdose
Atrazine
[35]Pesticides - Occurrence and Distribution in Streams and Ground Water
[36]Pesticides in the Groundwater of the US
[40]There Is No Denying This: Defusing the Confusion about Atrazine
[41]Pesticide Regulation amid the Influence of Industry
[42]EPA Pesticides: Reregistration Atrazine Updates
[43]Refined Ecological Risk Assessment for Atrazine
[44]Pest Control: Syngenta’s Secret Campaign to Silence Atrazine’s Critics
Additional Reading
[]Atrazine and amphibians: Data re-analysis and a summary of the controversy
[]Popular Pesticide Faulted for Frog’s Sexual Abnormalities
[]Studies conflict on common herbicide’s effects on frogs
Share